
- +86-13363869198
- weimiaohb@126.com

Sep . 19, 2025 11:30 Back to list
Semaglutide Powder-Hebei Weimiao|Diabetes Treatment&Weight Loss
Semaglutide, a groundbreaking pharmaceutical compound with the CAS number 910463-68-2, has emerged as a pivotal innovation in the treatment of type 2 diabetes and obesity. This article delves into the product's functionality, technical specifications, applications, and the company behind its production, Hebei Weimiao Import and Export Trade Co., Ltd.. By exploring its molecular structure, therapeutic potential, and industry relevance, this guide provides a detailed overview for professionals and consumers alike.
Product Overview: Understanding Semaglutide
Semaglutide is a pharmaceutical intermediate designed to mimic the action of glucagon-like peptide-1 (GLP-1), a hormone critical for regulating blood sugar levels. As a GLP-1 receptor agonist, it enhances glycemic control by stimulating insulin secretion and reducing glucagon release. Its unique chemical structure, featuring modifications such as the incorporation of 2-aminoisobutyric acid (Aib) and a fatty acid moiety, ensures prolonged efficacy and reduced metabolic degradation compared to older GLP-1 analogs like liraglutide.

Technical Specifications: Precision and Purity
The pharmaceutical intermediates market demands rigorous standards, and Semaglutide meets these with exceptional precision. Below is a detailed overview of its technical parameters:
| CAS No. | 910463-68-2 |
|---|---|
| Product Name | Semaglutide |
| Synonyms | Sermaglutide, Semaglutide fandachem, Semaglutide impurity, Sermaglutide USP/EP/BP |
| Molecular Formula | C187H291N45O59 |
| Formula Weight | 4113.57754 |
| Appearance | White solid powder |
| Purity | >98% |
| Storage | Dry, dark, 0–4°C (short-term) or -20°C (long-term) |
| Solubility | Soluble in DMSO |
| Shipping Condition | Ambient temperature as non-hazardous chemical |
Functional Mechanism: How Semaglutide Works
Semaglutide's efficacy stems from its structural modifications, which enhance its pharmacokinetic profile. The substitution of glycine with Aib at position 2 increases resistance to dipeptidyl peptidase-4 (DPP-4), an enzyme that degrades GLP-1. Additionally, the attachment of an 18-carbon fatty acid to the lysine residue at position 26 via a polyethylene glycol (PEG) spacer and γ-glutamic acid linker boosts its binding affinity to serum albumin. This dual modification results in a half-life of approximately seven days in humans, enabling once-weekly dosing—a significant advancement over daily GLP-1 agonists like liraglutide.
According to the National Institute of Standards and Technology (NIST), precision in molecular design is critical for pharmaceutical efficacy. Semaglutide's development exemplifies the importance of accurate chemical engineering in creating stable, long-acting therapeutic agents.
Applications: Beyond Diabetes
While Semaglutide is primarily approved for treating type 2 diabetes, its applications extend to weight management and neurodegenerative research:
- Weight Loss: Clinical trials demonstrate that Semaglutide significantly reduces body weight by suppressing appetite and delaying gastric emptying.
- Anti-Diabetic Therapy: It improves glycemic control by enhancing insulin secretion and reducing hepatic glucose production.
- Alzheimer's Disease Research: Preclinical studies suggest potential neuroprotective effects, though further research is needed.
The NIST emphasizes the role of standardized measurements in advancing drug development. Semaglutide's success in clinical trials underscores the importance of rigorous testing and validation in pharmaceutical innovation.
Company Profile: Hebei Weimiao Import and Export Trade Co., Ltd.
As a leading manufacturer of pharmaceutical intermediates, Hebei Weimiao Import and Export Trade Co., Ltd. has established itself as a reliable supplier of high-quality compounds. The company's commitment to quality is evident in its state-of-the-art production facilities and adherence to international standards.

Key Advantages of Hebei Weimiao
Hebei Weimiao's competitive edge lies in its comprehensive approach to product development and customer service:
- Quality Assurance: All products undergo 100% testing before delivery, with third-party verification available.
- Flexibility: Minimum order quantities (MOQs) are not restricted, accommodating both small-scale research and bulk production.
- Global Reach: The company ensures secure shipping through specialized logistics partners, with customs clearance handled efficiently.
- Customer Support: A dedicated team addresses inquiries promptly, offering samples for evaluation before bulk orders.
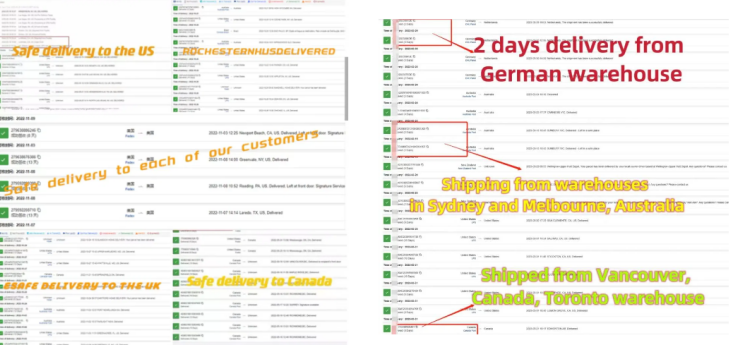
FAQ: Answers to Common Queries
Q: Are you a trading company or manufacturer?
A: We are a manufacturer, ensuring direct control over product quality and innovation.
Q: Can I request a sample?
A: Yes, samples are available for evaluation prior to bulk production.
Q: What payment methods do you accept?
A: Western Union, T/T, BTC, and Alibaba are among our accepted payment options.
Q: How is product quality ensured?
A: Every batch is rigorously tested, with third-party verification available upon request.
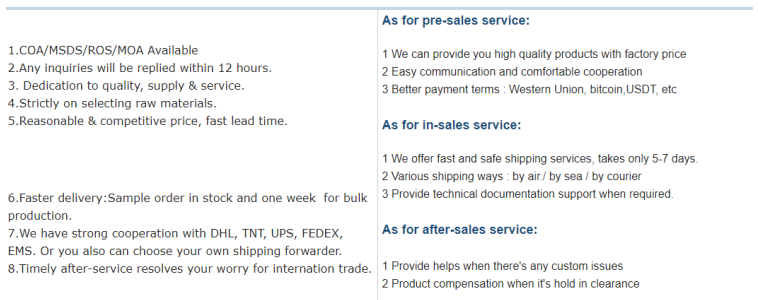
Conclusion: A Trusted Solution for Modern Medicine
Semaglutide represents a significant leap forward in the treatment of metabolic disorders. Its unique molecular design, coupled with the expertise of Hebei Weimiao Import and Export Trade Co., Ltd., ensures that healthcare providers and researchers have access to a reliable, high-purity compound. As the demand for advanced therapeutics grows, Semaglutide stands as a testament to the power of innovation in pharmaceutical science.
References
National Institute of Standards and Technology (NIST). (n.d.). Driving Innovation. Retrieved from https://www.nist.gov/
Hebei Weimiao Import and Export Trade Co., Ltd.. (n.d.). Product Specifications and Applications. Retrieved from https://www.weimiaobio.com
-
Benefits and Global Insights into Nature's Nutrition Turmeric Curcumin
NewsNov.24,2025
-
Curcumin BCM-95 – Enhanced Turmeric Extract for Superior Absorption & Health Benefits
NewsNov.23,2025
-
Discover the Health Benefits and Global Impact of Boswellia Curcumin Supplements
NewsNov.22,2025
-
BCM-95 Curcumin – Enhanced Natural Health Supplement | Weimiaobio
NewsNov.21,2025
-
Discover the Benefits of Super Bio Curcumin – Enhanced Absorption and Global Wellness
NewsNov.21,2025
-
Semaglutide Powder for Diabetes & Weight Loss - Hebei Weimiao
NewsNov.21,2025