
- +86-13363869198
- weimiaohb@126.com

មិថុនា . 11, 2024 10:10 Back to list
Medications Containing Semaglutide Marketed for Type 2 Diabetes or Weight Loss
What is semaglutide?
Semaglutide belongs to a class of medications known as glucagon-like peptide-1 (GLP-1) receptor agonists. It mimics the GLP-1 hormone that is released in the gastrointestinal tract in response to eating. One role of GLP-1 is to prompt the body to produce more insulin, which reduces blood glucose (sugar). GLP-1 in higher amounts also interacts with the parts of the brain that reduce appetite and signal a feeling of fullness.
Can semaglutide be compounded?
When a drug is in shortage, compounders may be able to prepare a compounded version of that drug if they meet certain requirements in the Federal Food, Drug, and Cosmetic (FD&C) Act. As of May 2023, Ozempic and Wegovy are both listed on FDA’s Drug Shortages list.
Are there concerns with compounded semaglutide?
FDA has received adverse event reports after patients used compounded semaglutide. Patients should not use a compounded drug if an approved drug is available to treat a patient. Patients and health care professionals should understand that the agency does not review compounded versions of these drugs for safety, effectiveness, or quality.
Additionally, FDA has received reports that in some cases, compounders may be using salt forms of semaglutide, including semaglutide sodium and semaglutide acetate. The salt forms are different active ingredients than is used the approved drugs, which contain the base form of semaglutide. The agency is not aware of any basis for compounding using the salt forms that would meet the FD&C requirements for types of active ingredients that can be compounded.
On April 27, 2023, FDA wrote to the National Association of Boards of Pharmacy expressing the agency’s concerns with use of the salt forms in compounded products. On Oct. 10, 2023, FDA sent additional letters to the National Association of Boards of Pharmacy and the Federation of State Medical Boards expressing similar concerns. The letters also explain the conditions under which compounded semaglutide products may be permissible under the law, and note that compounded drugs are not FDA-approved or evaluated for safety and effectiveness.
What should patients know about compounded semaglutide drugs?
Patients should be aware that some products sold as ‘semaglutide’ may not contain the same active ingredient as FDA-approved semaglutide products and may be the salt formulations. Products containing these salts, such as semaglutide sodium and semaglutide acetate, have not been shown to be safe and effective.
Patients should only obtain drugs containing semaglutide with a prescription from a licensed health care provider, and only obtain medicines from state-licensed pharmacies or outsourcing facilities registered with FDA.
High Quality Sermaglutide Powder Semaglutide CAS 910463-68-2
What should health care professionals know?
Health care professionals who are considering working with compounders to obtain semaglutide products should be aware that compounders may be using salt forms of semaglutide. FDA is not aware of any basis for compounding a drug using semaglutide salts that would meet federal requirements.
Has FDA found illegally marketed semaglutide online?
Yes. FDA vigilantly monitors the internet for fraudulent or unapproved products and has issued warning letters to stop the distribution of illegally marketed semaglutide. These drugs may be counterfeit, which means they could contain the wrong ingredients, contain too little, too much or no active ingredient at all, or contain other harmful ingredients.
Has FDA found counterfeit Ozempic in the U.S.?
FDA is aware and is investigating reports of counterfeit Ozempic being marketed in the U.S. The agency investigates any report of suspect counterfeit drugs to determine the public health risks and the appropriate regulatory response, and remains vigilant in protecting the U.S. drug supply from these threats.
How should patients protect themselves?
While we understand certain drugs are in short supply and patients are having difficulty obtaining their medication, FDA urges patients to obtain prescription drugs only from state-licensed pharmacies that are located in the U.S., where FDA and state authorities can assure the quality of drug manufacturing, packaging, distribution and labeling. FDA’s BeSafeRx campaign helps consumers learn about how to safely buy prescription medicines online. FDA recommends patients to talk to their doctor if they have questions about their medicines.
-
GS-441524 for White Liquid Factories: Boost Efficiency & Purity
NewsAug.04,2025
-
Premium Pharma Intermediates | AI-Optimized Synthesis
NewsAug.03,2025
-
GS-441524 White Liquid Production for Factories | AI-Optimized
NewsAug.02,2025
-
AI-Optimized CAS: 79099-07-3 Factories for High Yield
NewsAug.01,2025
-
Premium CAS 1451-83-8 Factory with GPT-4 Turbo | AI-Optimized
NewsJul.31,2025
-
Pharmaceutical Intermediates - AI-Optimized Synthesis & Purity
NewsJul.31,2025
មេសា . 19, 2024 10:38 Back to list
WHAT IS SEMAGLUTIDE?
WHAT IS SEMAGLUTIDE?
Semaglutide is a lipopeptide with a linear sequence of 31 amino acids. Like human Glucagon-like peptide-1 (GLP-1), it is used in combination with diet and exercise in the therapy of type 2 diabetes mellitus. It works as an anti-obesity agent, a neuroprotective agent, and an appetite depressant.
Semaglutide can be commercialized as a synthetic generic product after approval of an abbreviated new drug application (ANDA) or comparable applications with other regulatory authorities. The length and modifications make technical excellence and regulatory expertise a prerequisite for efficient filing and fast approval. High material quality and yield, robust processes including secure supply and innovative approaches at high-tech facilities will enable our customers to achieve their business goals.
ENSURING YOU A SMOOTH REGISTRATION PROCESS
Bachem has a long-standing track record in with successful registrations of highly purified synthetic peptide drugs of the glucagon family. Our regulatory intelligence keeps track of important changes in the relevant legislation. This enables us to be a leading global innovator in the field of glucagon and glucagon-like synthetic peptide drugs. Our services have been optimized to shorten timelines and of reduce complexity for our clients.
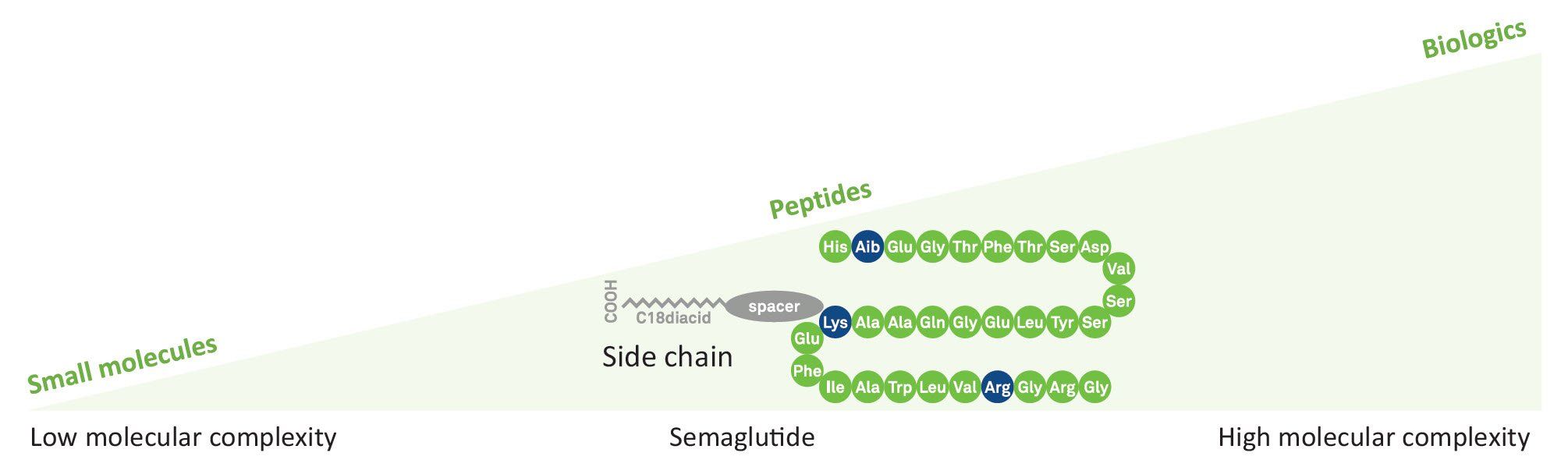
SCALABILITY, INNOVATION AND AUTOMATION
FROM SMALL TO LARGE-SCALE PRODUCTION WITH HIGH QUALITY
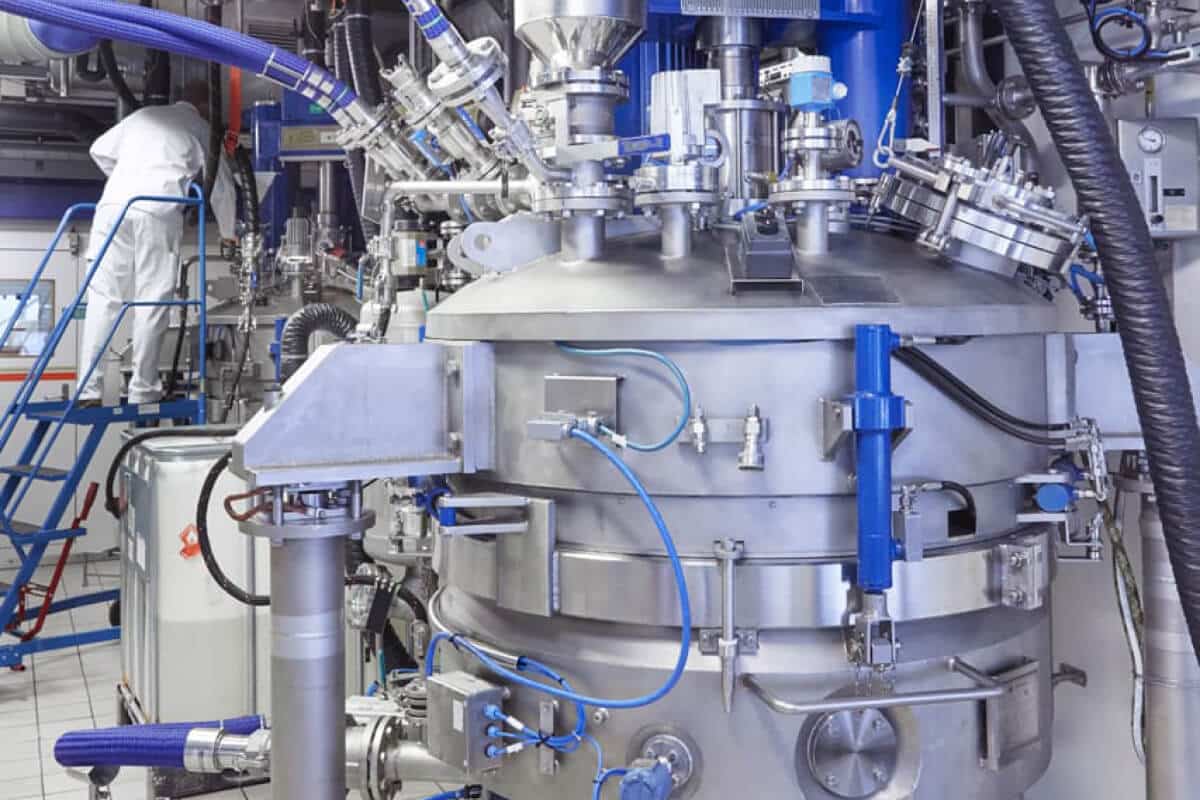
Our high-tech Good Manufacturing Practice (GMP) facilities based in Switzerland and the US, plus the commitment of our technical and scientific experts to quality, are the cornerstones for continuous compliance. We deliver small-scale to multi-kg active pharmaceutical ingredients (APIs) with impurities below 0.5%, identification and characterization of impurities above 0.10% using orthogonal analytical techniques.
High Quality Sermaglutide Powder Semaglutide CAS 910463-68-2
OPTIMIZED, AUTOMATED HIGH-YIELD PRODUCTION
Our long experience in complex APIs allows us to optimize the processes for high yields at outstanding quality. Our high level of process automation allows for cost-efficient and large-scale production resulting in excellent overall material purity (>99.5%). Innovative solutions like continuous chromatography let us use equipment and resources more efficiently and help us and our partners to achieve their commitment to sustainability and environment-friendly production. Our GMP sites in Switzerland and California comply with and surpass the most stringent regulations.
OUR TIMELINES FOR SEMAGLUTIDE
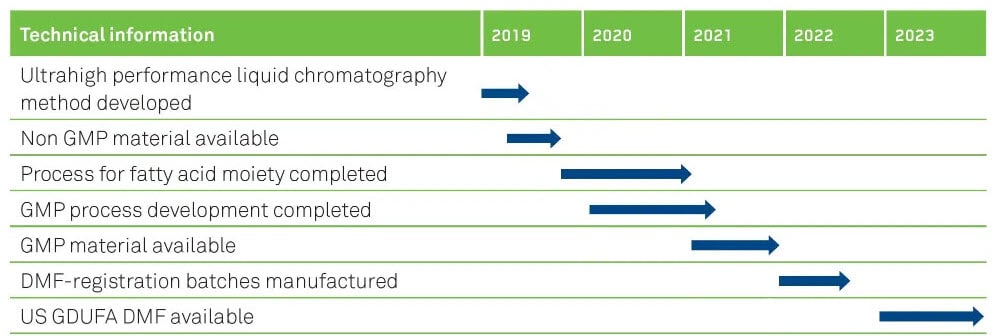
ROBUST PROCESSES AND SUPPLY SECURITY
Having our in-house building blocks for peptide synthesis as well as long-term cooperation with trusted suppliers ensures on-time production. Redundancy of multi-purpose equipment and facilities helps to mitigate risks in the supply chain, together with our stock of finished APIs and is the key for on-time product deliveries to our customers.
SERVICES
SEMAGLUTIDE IMPURITIES
The success of an abbreviated new drug application depends largely on the impurity profile of the synthetic peptide drug compared to the impurity profile of the reference listed drug (RLD) and the level of achieved “sameness”.
RELATED PRODUCTS (FOR RESEARCH PURPOSES ONLY)
Beyond GMP-grade semaglutide, we provide semaglutide in research grade, variants thereof and in different salt forms.
-
The Significance of Intermediate Pharma in Drug Development Is Crucial
NewsJun.24,2025
-
The Role of Organic Intermediates
NewsJun.24,2025
-
The Characteristics and Importance of Intermediate in Organic Chemistry
NewsJun.24,2025
-
Reaction Intermediate in Organic Chemistry Plays a Crucial Role in Organic Chemistry
NewsJun.24,2025
-
Pharmaceutical Intermediates Play a Critical Role in the Drug Manufacturing Process
NewsJun.24,2025
-
Bulk Drug Intermediates Are an Essential Component in the Production of Various Drugs
NewsJun.24,2025

