
- +86-13363869198
- weimiaohb@126.com

Dec . 05, 2024 23:16 Back to list
138-59-0 Suppliers and Manufacturers for Quality Chemical Solutions Availability
Understanding Manufacturers of 138-59-0
The chemical compound 138-59-0 is known as Triamcinolone acetonide, a synthetic glucocorticoid used primarily in the treatment of various inflammatory and autoimmune conditions. This powerful corticosteroid has established its significance in the pharmaceutical industry, leading to a growing demand for its manufacture and distribution. In this article, we will explore the manufacturers of 138-59-0, their role in the pharmaceutical supply chain, and the importance of quality and compliance in the production of this critical compound.
The Importance of Triamcinolone Acetonide
Triamcinolone acetonide is widely used in the treatment of conditions such as asthma, allergies, dermatitis, and arthritis. Its anti-inflammatory properties make it an effective option for managing symptoms associated with these ailments. The demand for this medication continues to rise due to increasing awareness of chronic diseases and a growing aging population that often requires long-term management of inflammatory conditions.
Manufacturers of 138-59-0
The manufacturing of 138-59-0 involves several key players in the pharmaceutical industry, including research and development organizations, active pharmaceutical ingredient (API) manufacturers, and contract development and manufacturing organizations (CDMOs). These manufacturers are responsible for producing high-quality Triamcinolone acetonide while ensuring compliance with stringent regulatory standards set by authorities such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
1. API Manufacturers These companies specialize in producing the active ingredients used in medications. Manufacturers of 138-59-0 must employ advanced chemical synthesis methods to ensure the efficiency and purity of the final product. This involves strict adherence to Good Manufacturing Practices (GMP), which guarantee that products are consistently produced and controlled according to quality standards.
138-59-0 manufacturers
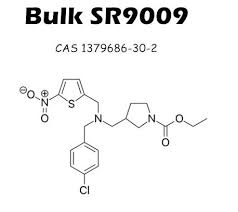
2. Regulatory Compliance Given the essential role that Triamcinolone acetonide plays in treatment regimens, manufacturers must navigate complex regulatory landscapes. Compliance with international regulations is imperative for securing market approval and maintaining the trust of health care providers and patients. Manufacturers of 138-59-0 must conduct extensive quality control and stability testing to ensure that their products meet or exceed the specifications required by regulatory bodies.
3. Contract Manufacturing Organizations (CMOs) Many pharmaceutical companies opt to partner with CMOs specializing in the production of specific APIs. These organizations can offer flexible capacity and advanced manufacturing capabilities, allowing pharmaceutical companies to focus on their core competencies, such as research and development or marketing. As a result, CDMOs play a significant role in the supply chain of Triamcinolone acetonide, helping meet the global demand for this important compound.
Quality Assurance in Manufacturing
Quality assurance is crucial in the manufacturing of 138-59-0. Manufacturers implement rigorous quality control measures to detect any deviations from production standards early in the process. This includes testing raw materials, in-process materials, and finished products to ensure they meet the required specifications for identity, purity, and potency.
Furthermore, manufacturers often invest in state-of-the-art technologies and analytical techniques, such as High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS), to ensure consistent product quality. These technologies not only facilitate precise measurements but also enhance the manufacturers' ability to meet regulatory requirements.
Conclusion
The landscape of manufacturing Triamcinolone acetonide, or 138-59-0, is a complex interplay of strict regulations, advanced technologies, and ongoing collaboration among various stakeholders. As the global demand for this important therapeutic agent continues to grow, manufacturers must prioritize quality, compliance, and innovation to ensure they can effectively contribute to the health and well-being of patients worldwide. The reliability and safety of Triamcinolone acetonide hinge on the commitment of these manufacturers to uphold the highest standards in their production processes, ultimately ensuring that this crucial medication remains available to those who need it.
-
High Quality SGT-163 CAS 1099-87-2 Supplier & Factory Reliable SGT-163 Manufacturer
NewsJun.10,2025
-
High Quality 3-Chloropyridine CAS 626-60-8 - Reliable Factories & Suppliers
NewsJun.10,2025
-
CAS 157115-85-0 Bulk Suppliers - High Purity & Low Prices
NewsJun.10,2025
-
High Purity PMK Ethyl Glycidate Manufacturer 99% Quality Supply
NewsJun.10,2025
-
Pure CAS 57-85-2 Testosterone Propionate Pharma Grade Supplier
NewsJun.09,2025
-
Premium Tadalafil CAS 171596-29-5 Suppliers & Factories
NewsJun.09,2025