
- +86-13363869198
- weimiaohb@126.com

Feb . 19, 2025 06:27 Back to list
lidocaine hydrochloride cas 73-78-9 factory
In the intricate world of pharmaceuticals, finding a reliable source for active pharmaceutical ingredients (APIs) can be a daunting task. Among these, Tadalafil, recognized by its Chemical Abstracts Service (CAS) number 171596-29-5, stands out due to its critical role in treating erectile dysfunction. A deep dive into the world of Tadalafil factories reveals a spectrum of qualities critical for quality assurance, regulatory compliance, and research and development.
Trustworthiness might be the most crucial aspect when sourcing Tadalafil, as it concerns the transparency and reliability of the factory. Trust is built through consistent product quality, transparent supply chains, and the ethical conduct of business operations. A trustworthy factory will provide comprehensive documentation ranging from certificates of analysis to material safety data sheets, demonstrating their commitment to safety and quality. Additionally, factories that prioritize fair trade practices and environmental sustainability further enhance their trust quotient. Sourcing Tadalafil from a reputable factory brings several advantages. The cost-efficiency of dealing with a factory directly often results in more competitive pricing, enabling pharmaceutical companies to allocate resources more effectively. Furthermore, having a direct relationship with the manufacturer ensures better communication, allowing for custom specifications tailored to meet specific product formulations or therapeutic needs. Contacting existing clients or partners of a Tadalafil factory can provide valuable insights to assess its credibility and the quality of its products. Customer reviews, testimonials, and third-party audits are invaluable resources for verifying a factory's standing in the industry. Additionally, exploring case studies or white papers authored by the factory can shed light on their commitment to advancement and innovation in Tadalafil production. Finally, a well-structured due diligence process involving visits to the factory premises, if feasible, enables first-hand assessment of the production environment and the standards upheld by the facility. Engaging with the technical teams can offer more profound insights into the operational expertise and the value system driving the organization. In conclusion, selecting a Tadalafil factory is a multifaceted decision that hinges on verifying the experience, expertise, authoritativeness, and trustworthiness of prospective partners. These factors collectively act as a beacon, guiding businesses toward quality, reliability, and success in a competitive pharmaceutical landscape. Opting for a factory that embodies these qualities assures not only the integrity of Tadalafil but also fortifies the foundation of a long-term strategic partnership.
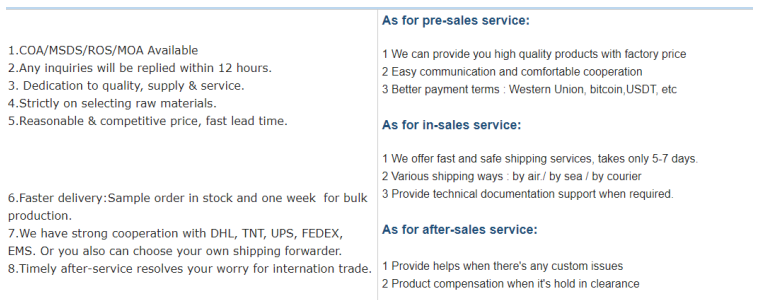

Trustworthiness might be the most crucial aspect when sourcing Tadalafil, as it concerns the transparency and reliability of the factory. Trust is built through consistent product quality, transparent supply chains, and the ethical conduct of business operations. A trustworthy factory will provide comprehensive documentation ranging from certificates of analysis to material safety data sheets, demonstrating their commitment to safety and quality. Additionally, factories that prioritize fair trade practices and environmental sustainability further enhance their trust quotient. Sourcing Tadalafil from a reputable factory brings several advantages. The cost-efficiency of dealing with a factory directly often results in more competitive pricing, enabling pharmaceutical companies to allocate resources more effectively. Furthermore, having a direct relationship with the manufacturer ensures better communication, allowing for custom specifications tailored to meet specific product formulations or therapeutic needs. Contacting existing clients or partners of a Tadalafil factory can provide valuable insights to assess its credibility and the quality of its products. Customer reviews, testimonials, and third-party audits are invaluable resources for verifying a factory's standing in the industry. Additionally, exploring case studies or white papers authored by the factory can shed light on their commitment to advancement and innovation in Tadalafil production. Finally, a well-structured due diligence process involving visits to the factory premises, if feasible, enables first-hand assessment of the production environment and the standards upheld by the facility. Engaging with the technical teams can offer more profound insights into the operational expertise and the value system driving the organization. In conclusion, selecting a Tadalafil factory is a multifaceted decision that hinges on verifying the experience, expertise, authoritativeness, and trustworthiness of prospective partners. These factors collectively act as a beacon, guiding businesses toward quality, reliability, and success in a competitive pharmaceutical landscape. Opting for a factory that embodies these qualities assures not only the integrity of Tadalafil but also fortifies the foundation of a long-term strategic partnership.
Latest news
-
Premium Pharma Intermediates | AI-Optimized Synthesis
NewsAug.03,2025
-
GS-441524 White Liquid Production for Factories | AI-Optimized
NewsAug.02,2025
-
AI-Optimized CAS: 79099-07-3 Factories for High Yield
NewsAug.01,2025
-
Premium CAS 1451-83-8 Factory with GPT-4 Turbo | AI-Optimized
NewsJul.31,2025
-
Pharmaceutical Intermediates - AI-Optimized Synthesis & Purity
NewsJul.31,2025
-
Top CAS: 79099-07-3 Factories & Wholesale Supplier from China
NewsJul.30,2025