- +86-13363869198
- weimiaohb@126.com

Jan . 30, 2024 14:39 Back to list
PMK ethyl glycidate-What is the synthesis and application of PMK ethyl glycidate?
What is the synthesis and application of PMK ethyl glycidate?
PMK ethyl glycidate, also known as 2-Oxiranecarboxylicacid, 3-(1,3-benzodioxol-5-yl)-2-Methyl-, ethyl ester, is a white solid at room temperature and pressure. It is commonly used as an organic synthesis intermediate and pharmaceutical intermediate, particularly in the synthesis and derivatization of drug molecules and bioactive molecules. With its epoxy unit, this compound can react with a range of nucleophilic reagents to prepare corresponding methyl piperonyl derivatives.

Figure 1: Image of PMK ethyl glycidate
GS-441524 适用于 Cat FIPV
Synthesis Method
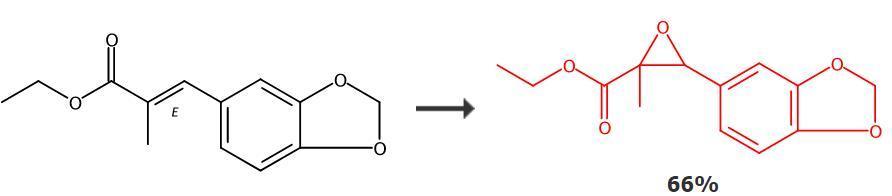
Figure 2: Synthesis route of PMK ethyl glycidate
In a dry reaction flask, the precursor compound of PMK ethyl glycidate (2 mmol) is dissolved in dichloromethane (20 mL), and the resulting reaction solution is cooled to 0°C. Slowly add m-chloroperbenzoic acid (863 mg, 5 mmol) to the mixture, and the reaction mixture is stirred at room temperature for 15 hours. After the reaction, extract the mixture with dichloromethane three times, combine all the organic layers, wash the organic layer with 5% NaHCO3 solution and brine, dry the organic layer with anhydrous sodium sulfate, filter off the drying agent, and concentrate the filtrate under vacuum to obtain the target product molecule.[1]
Application
PMK ethyl glycidate can be used as an organic synthesis intermediate and pharmaceutical intermediate. The reducible unit in its structure can react with organic amines to obtain ring-opened amine products.
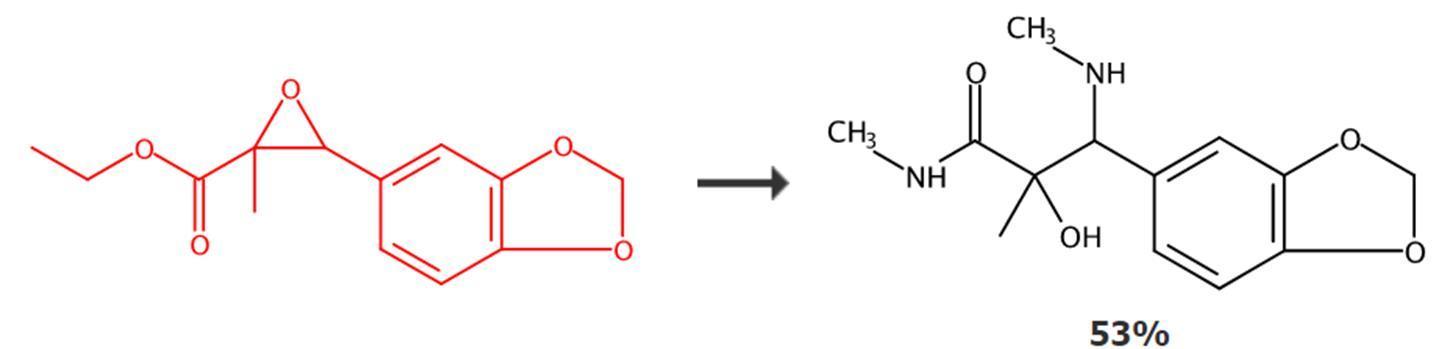
Figure 3: Application of PMK ethyl glycidate
In a dry reactor, dissolve PMK ethyl glycidate in methanol, then add a tetrahydrofuran solution of methylamine to the reaction mixture. Heat the reaction mixture to reflux and stir for several hours. After the reaction, neutralize the reaction system with formic acid, then extract the mixture with ethyl acetate three times, combine all the organic layers, dry the organic layer with anhydrous sodium sulfate, filter off the drying agent, and concentrate the filtrate under vacuum. The residue is purified by silica gel column chromatography to obtain the target product molecule.[1]
-
GS-441524 White Liquid Production for Factories | AI-Optimized
NewsAug.02,2025
-
AI-Optimized CAS: 79099-07-3 Factories for High Yield
NewsAug.01,2025
-
Premium CAS 1451-83-8 Factory with GPT-4 Turbo | AI-Optimized
NewsJul.31,2025
-
Pharmaceutical Intermediates - AI-Optimized Synthesis & Purity
NewsJul.31,2025
-
Top CAS: 79099-07-3 Factories & Wholesale Supplier from China
NewsJul.30,2025
-
High-Quality GS-441524 for White Liquid Type Factories & Suppliers
NewsJul.29,2025