
- +86-13363869198
- weimiaohb@126.com

Dec . 13, 2024 23:03 Back to list
Lidocaine Hydrochloride CAS 73-78-9 Supplier and Manufacturer Information
Lidocaine Hydrochloride An Overview of Manufacturer Practices
Lidocaine hydrochloride, with the Chemical Abstracts Service (CAS) number 73-78-9, is a widely utilized local anesthetic and antiarrhythmic agent. As a member of the amide local anesthetics, lidocaine is composed of a chemical structure that allows for effective nerve signal interruption, making it essential in various medical and dental procedures. Understanding its manufacturing process is crucial in ensuring both quality and effectiveness for its therapeutic uses.
The Importance of Quality in Manufacturing
Manufacturers of lidocaine hydrochloride must adhere to strict regulatory guidelines set forth by health authorities, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These organizations enforce Good Manufacturing Practices (GMP), which cover everything from sourcing raw materials to the final product's packaging. Compliance not only guarantees safety and efficacy but also builds consumer confidence in the product.
Raw Material Sourcing
The manufacturing of lidocaine hydrochloride begins with the procurement of raw materials. The primary constituent, 2,6-dimethylaniline, is a key ingredient and must meet high purity standards to minimize the risk of impurities affecting the final product. Manufacturers often establish long-term relationships with reputable suppliers, ensuring a consistent supply of quality raw materials.
The sourcing of other raw materials, including hydrochloric acid for the hydrochloride component, also requires diligence. Manufacturers need to conduct thorough evaluations of potential suppliers, analyzing their quality control processes, certification statuses, and overall reputations within the pharmaceutical industry.
Synthesis Process
The synthetic production of lidocaine hydrochloride involves several chemical reactions
. Typically, the first step is the N-alkylation of 2,6-dimethylaniline with 2-bromo-2-(diethylamino)ethyl chloride, which leads to the formation of lidocaine free base. Following this, hydrochloric acid is added to create lidocaine hydrochloride, facilitating its solubility in aqueous solutions.lidocaine hydrochloride cas 73-78-9 manufacturer
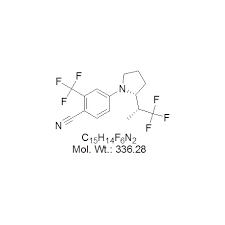
Controlling the conditions under which these reactions occur is vital. Temperature, pH, and reaction times must be carefully maintained to achieve the desired yield and purity. Advanced technologies, such as continuous flow reactors, are increasingly being implemented to improve efficiency and reduce batch-to-batch variability in the manufacturing process.
Quality Control Measures
Quality control is a critical aspect of lidocaine hydrochloride production. During manufacturing, samples are routinely taken at various stages for analysis to ensure they meet predetermined specifications for purity, potency, and presence of impurities. Analytical techniques commonly employed include High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), and Mass Spectrometry (MS).
Moreover, stability testing is carried out to determine the shelf life of the product, ensuring that lidocaine hydrochloride retains its efficacy over time. These rigorous assessments help in minimizing the risk of adverse effects when administered to patients.
Packaging and Distribution
Once manufactured and tested, lidocaine hydrochloride is packaged in sterile vials or ampules to maintain its stability and sterility. Manufacturers must ensure that all packaging materials are suitable for pharmaceutical use and comply with regulatory standards. Proper labeling is also crucial, as it provides necessary information regarding dosage, administration, and storage conditions.
Distribution channels are strategically chosen to guarantee that the product reaches healthcare providers efficiently. Manufacturers often collaborate with established pharmaceutical distributors to ensure that lidocaine hydrochloride is available where and when it’s needed.
Conclusion
Lidocaine hydrochloride, identified by the CAS number 73-78-9, plays a pivotal role in modern medicine, providing pain relief and cardiac care. The manufacturing process is complex and demands adherence to stringent quality standards to ensure patient safety and product efficacy. As advancements in technology continue to shape manufacturing practices, the future of lidocaine hydrochloride production promises not only to enhance efficiency but also to bolster the trust that healthcare professionals and patients place in this essential medication. Through stringent controls and commitment to quality, manufacturers strive to deliver lidocaine hydrochloride that meets the highest therapeutic standards.
-
Top CAS: 79099-07-3 Factories & Wholesale Supplier from China
NewsJul.30,2025
-
High-Quality GS-441524 for White Liquid Type Factories & Suppliers
NewsJul.29,2025
-
High-Quality Pharmaceutical Intermediates for Sale – Reliable Supply
NewsJul.29,2025
-
High-Quality Pharmaceutical Intermediates for Sale - Reliable Solutions
NewsJul.29,2025
-
High-Quality Pharmaceutical Intermediates Supplier for Global Market
NewsJul.28,2025
-
GS-441524 for White Liquid Type Factories – High Purity & Reliable Supply
NewsJul.28,2025