
Mercury is a chemical element with an atomic number equal to 80 and an atomic mass equal to 200.59 u. The element symbol of mercury is Hg, which can be expanded as hydrargyrum. The word hydrargyrum has been derived from two Greek words, namely hydor and argyros, meaning water and silver respectively. Mercury is generally known as liquid silver or quicksilver as it is silver in colour, lustrous, and is the only metallic element that tends to maintain its liquid state at room temperature and standard pressure conditions. Mercury is typically placed under the category of transition metal or post-transition metal. According to Roman mythology, the name mercury comes from the Roman god mercury who was known for his swiftness. Mercury is the only element that has retained its alchemical name as the modern chemical common name. Mercury is known to be discovered in and around 2000 BCE and India and China are known to be its discovering nations. The surface tension property of mercury is significantly high which enables it to form rounded beads of liquid. Mercury is one of the rarest elements that are available in Earth’s crust and account for approximately 0.08 parts per million. Mercury is usually found in the mineral cinnabar in the form of mercuric sulfide. To extract pure Mercury from the mineral cinnabar, the ore is crushed and heated in a furnace till the pure mercury gets vaporized. As compared to the conductivity of other metals, mercury is considered to be a relatively poor or mild conductor of heat and electricity. The freezing point of Mercury is equal to -38.8 C, while the boiling point is equal to 356 C. Mercury is immune to chemical reactions with most acidic elements. The electronic configuration of mercury causes it to form weak chemical bonds with other elements and behave like noble gases. The containers that are used to hold and transport mercury from one place to another are generally made up of iron as mercury tends to form amalgams with most metallic elements except iron. Using mercury in applications that make use of aluminium metal is generally not preferable as mercury quickly forms an amalgam with aluminium and disrupts its protective oxide layer, thereby causing the element to corrode.
Uses of Mercury
There are a number of daily life applications in our real life that make use of mercury. Some examples of such applications are given below:
1. Thermometer
Thermometers are one of the most prominent real-life applications that make use of the mercury element to note down and indicate the temperature values of objects or surroundings. Some of the daily uses of thermometers include measuring the body temperature of a living being, temperature inside an oven, temperature of meat, etc. A majority of thermometers rely on the properties of mercury for their prime operations. Mercury thermometers are used for domestic, commercial, industrial, as well as research applications. The high coefficient of expansion of mercury is one of the most important properties of mercury that make it fit to be used in thermometers. Likewise, other properties such as the liquid state at room temperature and the high boiling point of mercury help to expand the measurable temperature range of thermometers.

2. Fluorescent Lamps
A fluorescent lamp or a fluorescent tube typically comprises a glass tube, a phosphor layer, two electrodes, and a gas filling. When the voltage between the electrodes of the lamp or tube is maintained to the operable value, a gas discharge between the electrodes takes place. This discharge causes the charged particles or the electrons to move along the field generated by the electrodes and collide with the atoms of the gas. The collision between the atoms of the gas and the electrons causes the gas atoms to temporarily move into an excited state. The excited state is highly unstable which is why the atoms begin to lose energy and drop down to their original energy state after some time. While returning back to the original energy level, the atoms release energy generally in the form of UV light. UV light is not easily visible to humans, which is why a phosphor layer is laid on the inner side of the lamp or tube that tends to convert the ultraviolet light into visible light. The gas filling used in the fluorescent lamps or tubes is required to be selected very carefully according to the nature and type of the application. Experimentally, mercury vapour is proved to be a relatively more effective gas filing in fluorescent lamps than regular gas or a gas mixture. This is because of the correlation between light emitted by the mercury discharge and phosphors availability.

3. Float Valves
A float valve is a mechanical device that is generally used to detect, monitor, regulate, and control the fluid level in a container. A float valve is also known as a ball cock. The main operation of the float valve is to allow the flow of liquid into the container to a dedicated level and prohibit the flow after the volume of the fluid touches the threshold value. Float valves can be broadly categorised into two types, namely direct float control valves and pilot-operated float control valves. The material used for the construction of float valves is generally decided on the basis of the type of application where the device is required to be employed. For instance, a mercury float valve is best suited for high vacuum apparatus and the applications that deal with radioactive gases. The advantages of using a mercury float valve include the valve’s great conductance for gases and the ability of the valve to easily bear high-pressure difference values especially when the vapours of the organic compounds get completely eliminated or when the apparatus is employed in applications that deal with radioactive gases. Also, the mercury valve is comparatively compact and can be used s a needle valve.

4. Liquid Mirror Telescopes
A liquid mirror telescope is a telescope that makes use of a liquid substance as the reflective surface. The working of a liquid mirror telescope is quite similar to that of a regular telescope except for the fact that regular telescopes do not rely on liquid elements to collect the information points and build images of objects. The material that is generally used for the construction of a liquid mirror telescope is preferably mercury. The rotating dish of the liquid mirror telescopes is several meters in diameter and contains mercury in its molten form. When the rotating dish of the liquid mirror telescope is set to spin at a particular spinning rate, the liquid contained by it is forced to move in a circular direction with respect to the pivot of the disk and form a parabolic shape with raised edges. The gravitational force and the force generated due to inertia of rotation cause the liquid to spread on the surface of the disk evenly. The thickness of the layer of the liquid mirror is uniform throughout and is usually equal to 1-2 mm. When light radiation strikes the surface of the liquid mirror, it gets focused into the secondary non-liquid mirror, where the actual image gets formed. Liquid mirror telescopes are advantageous as they are comparatively lighter, less complex, simple to construct, and cheaper than the other traditional telescopes. The cost of a liquid mirror telescope is generally one-tenth part of the cost of a conventional glass mirror telescope. Mercury is preferred by liquid mirror telescopes as it remains molten at room temperature and absolute pressure conditions and is able to reflect over 75 per cent of the incident light. The reflection of light achieved by mercury is as good as silver. Also, the low gravity value of the moon causes mercury to spin at a significantly slower rate making it fit to be installed and used in outer space and other related applications.

High Quality 99% SR-9009 Cas 1379686-30-2
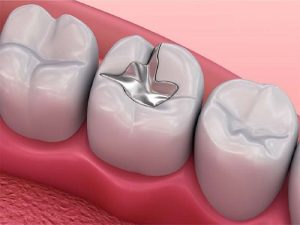
5. Dental Amalgams
Dental amalgam is nothing but a dental filling material that is generally used by dentists and medical personnel to fill the teeth cavities and cure tooth decay. The dental amalgam is basically obtained by forming a mixture of different metals such as mercury, silver, tin, copper, etc. In a dental amalgam, the element mercury is present in its natural liquid form, while the rest elements are present in powdered alloy form. About 50% of dental amalgam is composed of mercury, while the rest part contains the other metals. The composition of mercury in dental amalgams is significantly high as it tends to bind the alloy particles together and form a strong and solid filling that stays for a longer duration of time. The characteristic property of mercury that helps it maintain the element’s liquid state at room temperature improves the durability factor of dental amalgams.
6. Explosives
Mercury finds its prime importance and application in defence and security services to design war equipment and explosive devices. Mercury(II) fulminate is a chemical compound with a molecular weight equal to 284.63 u that can be used in the designing and formation of explosives. Such explosives usually get triggered and explode due to shock, friction, flame, heat, or any other type of exposure to temperature variation. It is also known as Fulminate of mercury or dry Fulminate of mercury,
7. Vaccines
Vaccines tend to form yet another example of the applications that make use of mercury. A variety of vaccines contain the chemical substance thimerosal, which is a mercury-based organic compound. The antimicrobial characteristics of thimerosal make it best suited to be used in various biological and drug products to prevent potentially life-threatening and harmful microorganisms. By weight, approximately 50% of thimerosal is composed of mercury. Also, other mercury-based organic compounds such as ethyl mercury, methyl mercury, etc. are used in vaccines and drugs. Methylmercury is an organic mercury compound that can be found in certain kinds of fish. The consumption of mercury-based compounds is required to be calculated and balanced as high exposure levels of such substances can be toxic.

8. Tattoo Ink
The red pigment used in most of the tattoo inks is generally derived from cinnabar. Cinnabar or cinnabarite is a naturally occurring rock that is primarily composed of mercury sulphide. This means that most of the red coloured tattoo inks contain mercury as a prime constituent. Due to the toxicity and other related natural characteristics of mercury, the use of mercury-based tattoo inks is considered somewhat risky; however, the side effect or the reaction caused due to tattoo inks entirely depend on the sanitary conditions and skin type of the user.

9. Contact Lens Solution
A contact lens solution is a disinfectant liquid that is generally used to rinse, clean, and moisturise lenses. Immersing the contact lenses in a contact lens solution helps keep the lenses safe from dirt, dust, germs, and microorganisms, thereby preventing any type of infections or side effects to the wearer. A contact lens solution primarily contains chemicals including hydrogen peroxide, boric acid, ascorbic acid, etc. Some of the contact lens solutions also contain a thimerosal chemical compound that is basically a mercuric derivative of thiosalicylic acid. In the case of contact lens solutions, mercury mainly tends to act as a preservative material.
10. Cosmetics
The existence of mercury in its natural sulphuric form is a great source of a red-orange pigment and can be derived easily by crushing the cinnabar rocks into powdered form. The pigment obtained as a result is completely organic and natural that can be used in various cosmetic goods and items. Also, mercury can also be used in cosmetics in its inorganic form. For instance, various skin lightening creams and lotions make use of inorganic ammoniated mercuric chloride and iodide. Here, mercury helps inhibit the formation of melanin in the skin, thereby resulting in a lighter skin tone. Other mercury compounds such as thiomersal and phenylmercuric salts can also be used in different cosmetic products. Thiomersal and phenyl mercuric salts tend to form the only mercury-based compounds that can be used in cosmetic products that are applied on or around the eyes. The use of mercury-based skincare and cosmetic products is quite dangerous as prolonged exposure and use of such products can lead to health issues and diseases such as skin rashes, discolouration, scarring, fungal and bacterial infections, etc. The side effect of using inorganic mercury-based skin lightening creams is kidney damage. This is the reason why organic, as well as inorganic mercury-based cosmetic products, should be used only after consultation or strict recommendation.

11. Vermilion
Vermilion tends to form yet another example of mercury-based products and applications in real life. Vermilion is generally obtained by crushing cinnabar mineral rock into smaller pieces and converting it into powdered form. The orangeish red pigment of vermilion is basically due to the presence of mercury in its sulphide form in the cinnabar rock. Vermilion is being used for different applications since the 8th century. Some of the examples of applications of vermilion include painting, dyeing, decoration, makeup, etc.

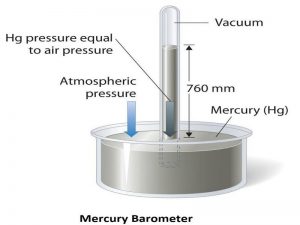
12. Barometer
A barometer is a scientific instrument and measuring device that helps the user measure and monitor atmospheric pressure. Mercury is usually preferred in barometers as the density of mercury is comparatively higher than the other fluids. The high-density value of mercury enables the user to easily detect change. Also, the high-density value of the fluid means a compact structure of the device, which is why a device that measures the atmospheric or barometric pressure value by employing mercury requires a smaller tube, while the device needs a relatively larger tube when other types of liquids are used. For instance, suppose two distinct barometers are required to measure the barometric pressure of a surrounding. One of the barometers uses water, while the other barometer uses mercury. In such a case, the barometer that uses water for pressure measurement is required to be constructed 13.6 times higher than the barometer that uses mercury to obtain the same value of pressure difference. This is because mercury is 13.6 times denser than water. Also, the low vapour pressure property of mercury aids its working in pressure measurement devices.
13. Eye Drops
Eye drops tend to form yet another application of mercury in the medical field. In eye drops, mercury acts as a preservative material to keep the chemical solution safe due to its antimicrobial characteristics; however, due to the potential toxicity of the substance, the use of such products is not usually preferred. The allergy-causing agents of mercury-based products tend to multiply at a significantly fast rate and cause moderate to severe health issues. The use of mercury and related elements in eye drops for children, senior citizens, and people with critically fragile immune systems is generally prohibited.


