
- +86-13363869198
- weimiaohb@126.com

Nov . 17, 2025 09:35 Back to list
GS-441524 for White Liquid Type Factories | GMP OEM Bulk
Inside Gs-441524 For White Liquid Type Factories: real-world specs, process, and buyer tips
If you’re sourcing GS-441524 liquid for feline FIP programs, the conversation quickly moves past buzzwords to sterility, assay consistency, and—let’s be honest—who can deliver repeatable batches without drama. I’ve toured a few plants and spoken with QC leads; the gap between marketing and the fill line can be wider than most buyers expect.
Product snapshot and formats
From Weimiao Bio (Origin: Room 1727, Dongyiyuan International, Xinyi Road, Shijiazhuang, Hebei), “GS-441524 For Cat FIPV” is offered as a white liquid type and in pill formats. Many customers say the liquid route is preferred in induction phases, with tablets used later for maintenance—your protocol may differ, of course.
| Form | Strength / Pack | Typical Excipients (≈) | Notes |
|---|---|---|---|
| Liquid (white) | 20 mg/mL, 30 mg/mL; 6/7/8/10 mL vial | Water for injection, buffer, stabilizers | For rapid titration; check osmolality and pH |
| Pills | 5, 10, 20, 25, 40, 50, 60 mg (custom on request) | Lactose-free base, binders (varies) | Batch uniformity key for home dosing |

Process flow in Gs-441524 For White Liquid Type Factories
- Materials: API GS-441524 (≥98–99% by HPLC), WFI, pharm-grade excipients.
- Methods: Dissolution under nitrogen, pH adjustment (≈6.8–7.4), 0.22 μm sterile filtration.
- Filling: Aseptic vial filling in ISO 5 with ISO 7 background; laser or crimp seals.
- Testing standards: USP <71> Sterility, USP <85> Endotoxins (target <0.5 EU/mL), USP <788> Particulates, assay by HPLC/LC–MS.
- Stability: ICH Q1 accelerated/long-term; typical unopened shelf life ≈ 18–24 months (real-world may vary).
- Industries served: Veterinary clinics, shelters, research labs, reseller networks.
Example in-house test data (typical targets)
Assay 99.1% (HPLC), endotoxin 0.12 EU/mL, pH 7.1, osmolality 300 mOsm/kg, sterility pass at 14 days. I guess that’s what you’d want to see on a COA—always request one lot-specific.
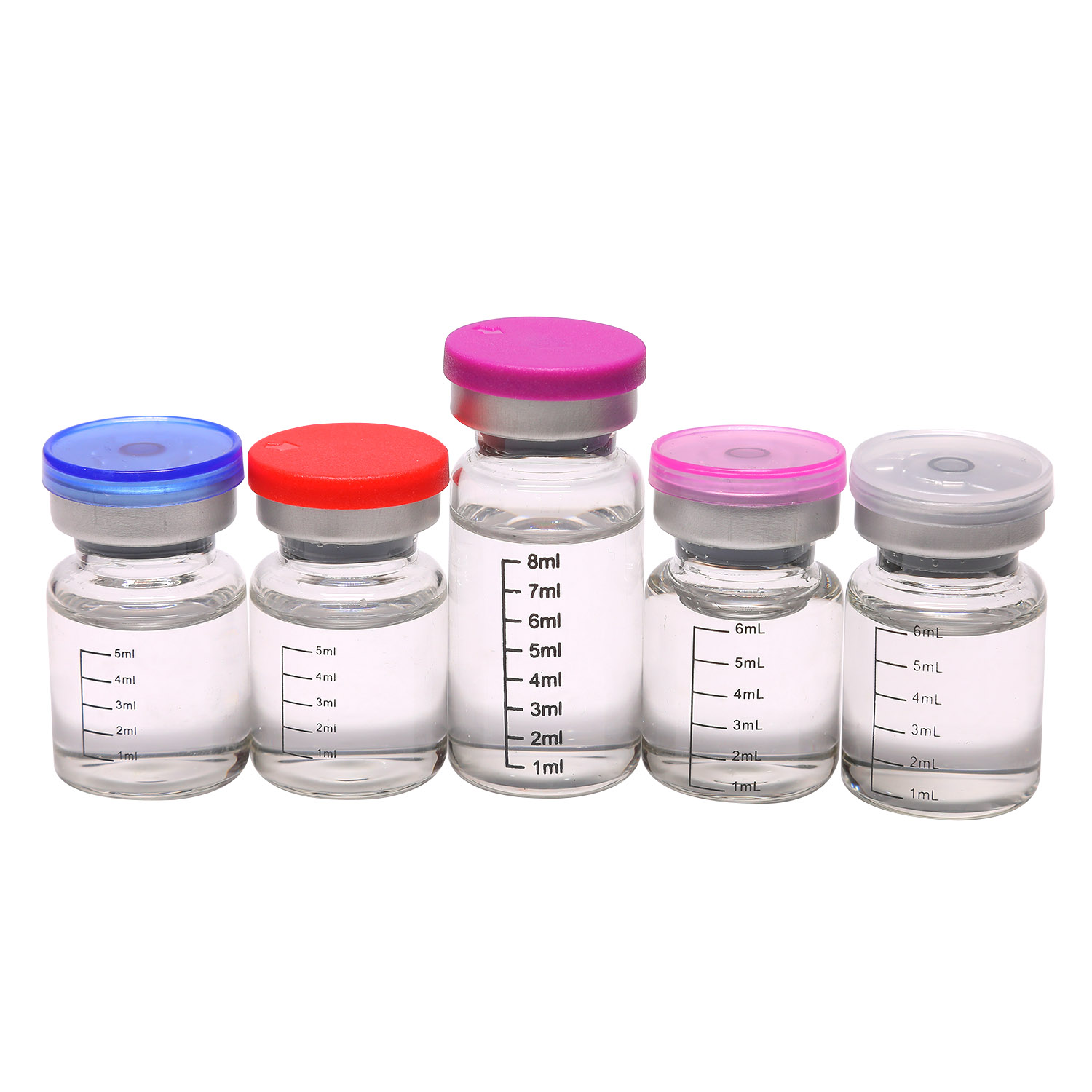
Application scenarios and feedback
Use spans induction dosing in suspected wet/dry FIP, maintenance therapy, and clinic-to-home transitions. Many buyers mention smoother dosing with 30 mg/mL for larger cats to reduce injection volume. Storage 2–8°C is common; in-use stability after puncture ranges 7–28 days depending on preservative—check the label.
Vendor comparison (quick buyer’s lens)
| Vendor | GMP/ISO signals | MOQ | Customization | Lead time | QC docs |
|---|---|---|---|---|---|
| Weimiao Bio (Shijiazhuang) | ISO 9001/GMP-like processes (ask for certificates) | Flexible | Vial size, mg/mL, pills | Around 7–14 days | COA, sterility, endotoxin |
| General trading supplier | Variable | Medium | Limited | 10–20 days | Basic COA, sometimes incomplete |
| Clinic compounding lab | Pharmacy QA standards | Low | High (patient-specific) | 1–5 days | Batch records, labels |

Customization and case notes
Custom mg/mL, vial fill volumes (6–10 mL), and tablet strengths are common asks in Gs-441524 For White Liquid Type Factories. Two quick snapshots: (1) A shelter network cut wastage by switching from 10 mL to 7 mL vials; (2) A referral clinic reported fewer injection complaints after moving from 20 mg/mL to 30 mg/mL (smaller volumes, same dose). Not scientific, but intriguingly consistent feedback.
What to request before PO
- Lot-specific COA: assay, sterility (USP <71>), endotoxin (USP <85>), pH/osmolality, particulates (USP <788>).
- Stability summary (ICH Q1) and storage conditions.
- Cleanroom classification and aseptic validation records.
- Recall/complaint procedure and after-sales replacement policy.
In short, Gs-441524 For White Liquid Type Factories that publish transparent QC and permit small pilot lots tend to win repeat business. Actually, it’s the boring paperwork that tells you if the fills will be boringly reliable.
Citations
- Pedersen NC. Black JJ, et al. Efficacy of GS-441524 for feline infectious peritonitis. J Feline Med Surg, 2019.
- Gandolfi B, et al. Feline infectious peritonitis treatment observations with nucleoside analogs. Animals, 2021.
- USP General Chapters: <71> Sterility, <85> Bacterial Endotoxins, <788> Particulate Matter.
- ICH Q1 Stability Testing of New Drug Substances and Products; ICH Q7 GMP for APIs.
-
CAS: 79099-07-3 Factories | GMP Stock, OEM, Fast Delivery
NewsNov.17,2025
-
CAS: 79099-07-3 Factories | GMP Quality, Factory Prices
NewsNov.17,2025
-
GS-441524 for White Liquid Type Factories | GMP OEM Bulk
NewsNov.17,2025
-
CAS: 79099-07-3 Factories | GMP Bulk Supply, Fast Shipping
NewsNov.17,2025
-
gs-441524 for white liquid type factories | GMP & fast ship
NewsNov.17,2025
-
High-Purity cas 1451-83-8 factory | GMP Bulk, Fast Delivery
NewsNov.17,2025