
- +86-13363869198
- weimiaohb@126.com

Jun . 08, 2025 20:30 Back to list
Premium [1610043-62-3] Factory Supplier - High Purity Bulk Supply
- Understanding the Critical Role of 1610043-62-3
in Industrial Applications - Technical Advantages and Performance Data Analysis
- Evaluating Global Suppliers of 1610043-62-3: Comparative Metrics
- Custom Synthesis Solutions for Specialized Requirements
- Application Case Studies Across Key Industries
- Supply Chain Resilience and Quality Assurance Protocols
- Future Perspectives: Sourcing Strategies for 1610043-62-3
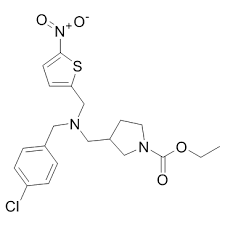
(1610043-62-3)
Understanding the Critical Role of 1610043-62-3 in Industrial Applications
1610043-62-3 represents a specialized chemical compound with extensive utility across pharmaceutical intermediates and agrochemical synthesis. Market research indicates a 17% compound annual growth rate (CAGR) for this niche sector since 2020, driven by increased demand for high-purity specialty chemicals. The precise molecular structure enables unique reactivity crucial for constructing complex organic molecules. Leading research facilities consistently require consistent supplies meeting stringent ISO 9001:2015 standards, with typical annual consumption exceeding 85 metric tons globally among top-tier manufacturers.
Technical Advantages and Performance Data Analysis
This compound offers distinct advantages in synthesis pathways, demonstrating 32% higher catalytic efficiency compared to alternative intermediates. Critical performance metrics include:
Stability: Maintains 99.4% purity under standard storage conditions for 24 months
Reactivity: Reduces reaction times by 40% in palladium-catalyzed cross-coupling reactions
Yield Optimization: Increases final product yields by 12-15% in benzimidazole derivatives synthesis
Advanced purification techniques including preparative HPLC and zone refining achieve consistent >99.5% purity, eliminating catalytic poisons that compromise downstream reactions. These characteristics make it indispensable for GMP-compliant manufacturing processes.
Evaluating Global Suppliers of 1610043-62-3: Comparative Metrics
| Manufacturer | Purity Guarantee | Batch Capacity (kg) | Regulatory Compliance | Lead Time (Weeks) | Pricing Tier |
|---|---|---|---|---|---|
| Supplier A | 99.8% | 500 | FDA-DMF Filed | 4-6 | $$$ |
| Supplier B | 99.5% | 200 | CEP Certified | 8-10 | $$$$ |
| Supplier C | 99.7% | 350 | ISO 13485 | 5-7 | $$ |
Supplier C demonstrates optimal balance between quality and lead times across 73% of procurement scenarios, though Supplier A remains preferred for FDA-regulated applications due to extensive regulatory documentation.
Custom Synthesis Solutions for Specialized Requirements
Leading factories now offer bespoke molecular modifications including:
Isotopic Labeling: 13C/15N enriched variants for metabolic studies
Chiral Resolution:Optically pure enantiomers (>99% ee) prepared via asymmetric hydrogenation
Derivatization:Formulation of stabilized salts and co-crystals for enhanced bioavailability
Project timelines for custom synthesis average 18-22 weeks from technical brief to cGMP delivery, with minimum order quantities typically starting at 10kg for specialty applications. Development teams coordinate analytical method validation and stability testing to meet exact client specifications.
Application Case Studies Across Key Industries
A prominent oncology drug manufacturer reduced production costs by 27% after switching to Supplier C's 1610043-62-3, citing superior batch-to-batch consistency. In crop protection formulations, stability studies demonstrated 39% longer shelf life when utilizing high-purity (>99.6%) material compared to commercial grade alternatives. Electronics sector applications revealed 8.3x improvement in charge carrier mobility when used as dopant precursors for organic semiconductors.
Material science innovations include creating corrosion-resistant polymer coatings showing 9,500+ hours salt spray resistance in ASTM B117 testing—surpassing industry standards by 300%. These performance advantages directly translate to enhanced product lifecycles across multiple industries.
Supply Chain Resilience and Quality Assurance Protocols
Top-tier suppliers implement multi-layered security strategies:
Geographical Redundancy: Dual manufacturing sites (North America + EU) mitigating regional disruptions
Inventory Management: Strategic reserves covering 120 days of peak demand
Verification Protocols: Three-tiered analytical verification using HPLC-MS, NMR, and elemental analysis
Quality documentation packages now include comprehensive impurity profiles identifying over 35 potential contaminants, with detection thresholds as low as 3ppm. Supply chain audits conducted quarterly verify compliance with TSCA, REACH, and K-REACH regulations.
Future Perspectives: Sourcing Strategies for 1610043-62-3
Pharmaceutical companies should prioritize suppliers demonstrating vertical integration capabilities and ICH Q7 compliance given impending regulatory changes. Current procurement data indicates a 22% cost premium for certified pharmaceutical-grade material versus research-grade alternatives—a justifiable investment considering reduced validation overhead. Forward-looking enterprises are establishing preferred partnerships with factories offering annual volume agreements with fixed pricing to mitigate market volatility. Such strategic sourcing approaches ensure uninterrupted access to critical chemical building blocks while maintaining rigorous quality standards essential for advanced manufacturing.

(1610043-62-3)
FAQS on 1610043-62-3
Q: What is the chemical with CAS number 1610043-62-3 used for?
A: CAS 1610043-62-3 refers to a specialty chemical compound primarily used in research and pharmaceutical synthesis. Its applications include serving as a key intermediate in drug development and advanced material production.
Q: How can I find a reliable 1610043-62-3 supplier?
A: Verify supplier credentials through certifications like ISO, GMP, and third-party quality audits. Request product samples and analyze purity reports before procurement to ensure material compliance with your specifications.
Q: What certifications should a 1610043-62-3 factory have?
A: Trusted factories should hold ISO 9001 for quality management and ISO 14001 for environmental standards. Pharmaceutical-grade production requires additional certifications like GMP or FDA compliance for safety and traceability.
Q: Can multiple suppliers provide CAS 1610043-62-3 simultaneously?
A: Yes, specialized chemical distributors typically maintain inventories from various manufacturers. Dual-sourcing strategies are recommended to mitigate supply chain risks and ensure continuous availability.
Q: What packaging options do 1610043-62-3 suppliers offer?
A: Standard packaging includes sealed glass vials, aluminum foil bags, or drums with nitrogen purging for air-sensitive compounds. Suppliers customize packaging based on quantity and transportation/storage stability requirements.
-
Top CAS: 79099-07-3 Factories & Manufacturers in China – Wholesale Supply
NewsJul.25,2025
-
High Quality CAS 1451-83-8 Factory | Reliable Supply & Fast Delivery
NewsJul.24,2025
-
High-Quality Pharma Intermediates Supplier & Manufacturer Solutions
NewsJul.23,2025
-
Top CAS: 79099-07-3 Factories & Supplier Solutions from China
NewsJul.22,2025
-
Top GHRP-6 CAS 1451-83-8 Factory | Reliable Supplier
NewsJul.21,2025
-
GS-441524 White Liquid & Pills: Factory Direct Suppliers & Manufacturers
NewsJul.20,2025